Contact angle and surface tension. The two terms are never far apart. Run a Google search of "contact angle" right now and see for yourself. You'll often see the terms mentioned within a breath of one another because contact angle and surface tension are two approaches to surface analysis - the study of how a surface interacts with other materials or components. Generally speaking, there is also a relationship between the two measurements, one that tends not to be very clearly explained in other resources. I hope to remedy that today.
To begin, we have to start by defining some basic terms related to this discussion of surface analysis.
Surface Tension
Surface tension is the cohesive force of molecules at the surface of an element attracting toward one another to take up the least possible surface area. In even simpler terms, it measures how much force it takes to keep a liquid together.
Contact Angle
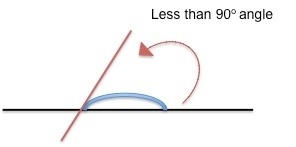
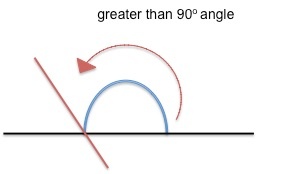 Contact angle is measured as the angle where a liquid or a vapor (but most often a liquid) interacts with a solid surface. A high contact angle (pictured right) indicates that the surface has low wetting - that is, the liquid droplet will not spread very much onto the surface. A low contact angle (left, below) indicates that the surface is high wetting, meaning that the water droplet spreads out more on the surface.
Contact angle is measured as the angle where a liquid or a vapor (but most often a liquid) interacts with a solid surface. A high contact angle (pictured right) indicates that the surface has low wetting - that is, the liquid droplet will not spread very much onto the surface. A low contact angle (left, below) indicates that the surface is high wetting, meaning that the water droplet spreads out more on the surface.
Contact angle analysis is used to measure the quality of a solid surface. Surface tension analysis is used to measure the quality of a liquid. Clear so far?
There's one final term we need to define before we move on: surface energy. Surface energy is the amount of intermolecular force created at the surface of a material, and it determines the amount of attractive or repulisve force a surface can exert on another material.
Types of Surface Tension
Here's where the other resources can be a bit murky - when you talk about surface tension in relation to contact angle, there are two types of surface tension at play: the surface tension of the liquid, and the interfacial tension between the liquid and the solid.
Interfacial tension is defined as the measure of adhesive force between the liquid phase of one substance and the liquid, solid, or gas state of another substance. Interfacial tension is high on a hydrophilic surface and low on a hydrophobic surface.
Wettability
Think of it this way - a high wetting surface has a surface energy that creates a strong attractive force to pull the liquid droplet down, causing it to spread out. This is known as wettability. This surface energy is stronger than the surface tension of the liquid's molecules that would normally keep it in droplet form. The surface tension of the liquid doesn't change, but rather the surface energy of the solid is stronger than the liquid's surface tension and overpowers it, causing the liquid to spread out over the solid surface. Low contact angle = high surface energy and high interfacial tension.
For a low wetting surface, the surface energy is weaker than the surface tension of the liquid, meaning that the liquid can better keep its droplet shape. The interfacial tension between the solid and liquid is low because the interaction between the two is not as strong. High contact angle = low surface energy and low interfacial tension.
Who Cares?
Why does this relationship between contact angle and surface tension matter? There is a wide range of applications that rely on this relationship. Think of the adhesion of ink to paper - the surface energy of the paper must be strong enough to overpower the surface tension of the ink and draw it down to spread out on the paper. Paint is another good example - the surface can't be too wettable, or else the paint will just bead up (thanks to the paint's surface tension being stronger than the surface energy) and drip off of the surface rather than spread out and adhere to it.
We'll be talking more about contact angle in blog posts to come because we've just added a new Contact Angle Meter to our product line. Check it out!
In the meantime, reply in the comments section of this post if you've got any questions. Happy testing!
-Amanda
P.S. Don't forget to subscribe at the top of the page to get automatic updates whenever we post a new article!
P.P.S. Checkout the infographic about wetability.


